TGGENCO Download Hall Ticket 2024 Assistant Engineer, Chemist : Telangana State Power Generation Corporation
Organisation : TGGENCO Telangana State Power Generation Corporation Limited
Recruitment Name : Assistant Engineers and Chemist
Announcement : Download Hall Ticket
Exam Date : 14th July 2024
Website : https://tggenco.com/TGGENCO/getInfo.do
How to Download TGGENCO Assistant Engineer & Chemist
Hall Ticket?
Online Computer Based Test (CBT) for the post of Assistant Engineers and Chemist is re-scheduled and will be conducted on 14.07.2024. To Download Hall Ticket follow the below steps
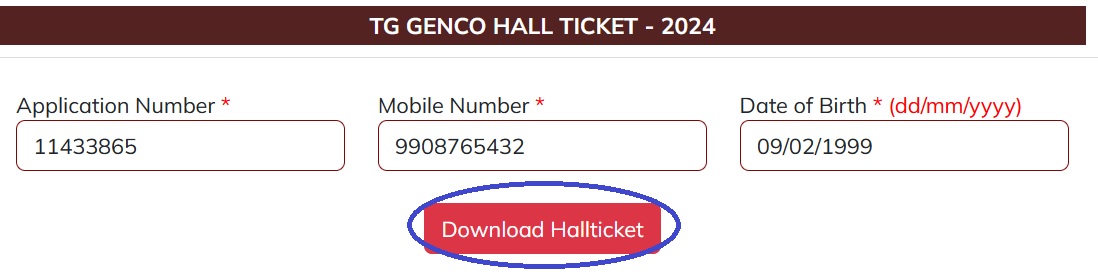
Steps :
Step-1 : Go to the link http://myapplication.in/TGGENCO/TGGENCO/TGGENCO_HALLTICKET_2024.aspx
Step-2 : Enter Application Number
Step-3 : Enter Mobile Number
Step-4 : Enter Date of Birth (dd/mm/yyyy)
Step-5 : Click on “Download HallTicket” Button.
Related / Similar Admit Card : School Education Telangana Download Hall Ticket 2024 TS DSC Teacher
Syllabus For Chemist TSGENCO
Section –A Total 80 Marks
1) COORDINATION CHEMISTRY:
Metal – ligands bonding – valance bond – crystal field and molecular orbital theories of stability of coordination compounds – its determination – chelate compounds – Applications of magnetic moment data for the determination of oxidation states, bond type and stereochemistry.
2) ORGANOMETALIC CHEMISTRY:
Chemistry of organo metallic compounds of Li, Na, Mg, Zn, Hg, Al, Pb, Sn
3) BIOINORGANIC CHEMISTRY:
Importance of metal ions in biological systems – Oxygen transport and storage – Structure and function of Haemoglobin and Myoglobin – Vitamin B6 catalyzed reactions – Transamination, Decarboxylation and Dealdolation reactions – Role of metal ion in model system studies.
4) Thermal Methods, AAS, AES, ICP-AES:
Thermal methods of analysis: Thermogravimetry – Differential Thermal Analysis and Differential Scanning Calorimetry, instrumentation – Methodology of TG, DTA and DSC.
Atomic Absorption Spectroscopy (AAS): Principles of AAS, Instrumentation – flame AAS and furnace AAS, resonance line sources, sensitivity and detection limits in AAS, interferences – chemical and spectral, evaluation methods in AAS and application in qualitative and quantitative analysis.
Atomic Emission Spectroscopy (AES): Principles of AES, Instrumentation, evaluation methods, Application in quantitative analysis. Inductively Coupled Plasma – Atomic Emission Spectroscopy (ICP-AES): Limitations of AES, Principles of plasma spectroscopy, plasma as an excitation source. Inductively coupled plasma source, ICP-AES – Instrumentation.
5) QUANTUM CHEMISTRY:
Black body radiation – Planks concepts of quantization, wave particle duality and uncertainty principle, Eigen functions and eigen values, operations – operator algebra, communication of operators well behaved functions, Normalized and orthogonal function. Particle in a box – one-dimensional and three-dimensional – energy and wave functions of particle in a box – degeneracy application to the spectra of conjugated molecules.
6) THERMODYNAMICS:
First law of thermodynamics and its applications – second law of thermodynamics and its applications – definition of free energy – clapeyron and clausius – clapeyron equations –activity and activity coefficient.
7) CHEMICAL KINETICS :
Collision and transition state theory, Unimolecular reactions and Lindamanns theory, opposing , parallel and consecutive reaction chain reaction, H2 – Br2 reactions, its rate law General acid catalysis ( hydrolysis of ester ) general base catalysis (aldol reaction) enzyme kinetics – Michaelis – Minton Kinetics.
8) ELECTROCHEMISTRY:
Theories of strong electrolytes – interionic interaction and their experimental manifestation – Debye Huckel theory of activity coefficients – measurement of activity coefficient and experimental verification of Limiting law – electrochemical cells and Nernst equation. Origin of back e.m.f. in different type of cells
9) SURFACE CHEMISTRY:
Liquid interfaces – stability of the interface – Gibbs Adsorption equation – surface films and Experimental methods for their study – Gas – solid adsorption isotherms and surface area measurements.
10) SPECTROSCOPY:
Microwave spectroscopy – selection rules, reduced mass bond length rotational spectra of diatomic molecules energy equation. IR: Anhormonic nature of vibration spectra – group frequencies – calculations, of force constant of a diatomic molecules from vibrations spectra – group frequencies – identification of functional groups – hydrogen bonding – Cs – Trans isomerisation. NMR: Basic principle Larmor frequency chemical shift including calculations, spin-spin splitting – first order spectra – double resonance – applications of PMR spectroscopy in structure determination
11) STEREOCHEMISTRY:
Symmetry of molecules and criterion for optical activity different configurationally notations asymmetric induction stereo specific reactions – activity of nitrogen compounds – dynamic enantiomerism conformational analysis of cyclic and acyclic molecules – cis, trans isomerism.
12) REACTION MECHENISM:
Electronic effects, addition to c=c and c=o. Aliphatic and aromatic nuclephilic and electrophilic substitution reactions. Neighbouring group participation. Different types of Elimination reaction. Various methods of studying the reaction mechanisms. Generation, detection and stability of reactive intermediates.
13) CARBOHYDRATES:
Structure and configuration of monosaccharide’s – reducing and non reducing disaccharides – polysaccharides.
14) AROMATICITY:
Criteria – different rules molecular orbital approach – annulenes – aromatic cations and anions – metallosenes – nonbenzionoid aromatics.
15) Introduction to analytical chemistry:
Safety in laboratory; Analytical balances – types, care & use, weights and reference mass; Water for lab use – different grades and methods of purification of water; Reagents and Standard solutions – Expression and units of concentrations, Classification of Errors-Systematic & random errors, identification and reduction of these errors; Significant figures; Qualitative and Quantitative analysis; Statistical functions and reliability of results;
Section –B Total 20 Marks.
General Awareness and Numerical Ability:
i) Analytical & Numerical Ability
ii) General Awareness
iii) English
iv) Related to Telangana Culture, Movement
v) Computer Knowledge

Written Examination Instructions to Candidates
1. The test is of two hours duration. The date and time will be indicated on the Hall ticket. Candidates should reach the test center in time. Candidates will be allowed in to the examination hall half an hour before the scheduled starting time. Candidates will not be allowed into the examination hall after the test has started and will not be permitted to leave examination hall before the closure of test time under any circumstances.
2. The test will be of objective type with multiple-choice questions with only one answer being correct among the four alternatives suggested.
3. A separate O M R (Optical mark Reader) answer sheet with the carbon impression paper will be provided to the candidates. The candidate has to indicate his response to each question by darkening the appropriate bubble with a Black Ball Point pen. No corrections with white fluid will be permitted.
4.The candidate has to bring a good quality Black Ball point pen to the examination hall.
5. The candidate has to handover the original OMR Sheet to the invigilators in the examination centre and is however permitted to take away the duplicate OMR Sheet (the carbon impression paper) along with question paper after the examination. If any candidate in violation of the above instructions takes away the original OMR Sheet, his/her candidature to the recruitment will be rejected besides invocation of penal provisions including debarment of the candidature for all future recruitments to be conducted by the TSGENCO.
6. The candidate has to follow meticulously all the instructions given on the question paper booklet and OMR Answer Sheet, else his answer sheet may not be valued.
7 Usage of Calculators/mathematical tables is not permitted. Candidates should not bring cell phones or any other electronic gadgets to the examination hall.
8. The provisional key will be publicized one day after the examination.
9. If any objections on the provisional key, they can be raised in the website .

Recent Comments